Bexotegrast at 320 mg was well tolerated up to 40 weeks of treatment with
no treatment-related severe or serious adverse events
320 mg cohort demonstrated improvement in liver stiffness by transient elastography
at Week 24 compared to placebo
Statistically significant improvement in alkaline phosphatase (ALP) levels
over 24 weeks compared to placebo
Continued improvement in hepatocyte function and bile flow by contrast
MRI imaging observed from Week 12 to Week 24
FDA guidance provided clarity on next steps of PSC development program
SOUTH SAN FRANCISCO, Calif., July 15, 2024 (GLOBE NEWSWIRE) -- Pliant Therapeutics, Inc. (Nasdaq: PLRX) today announced positive 24-week data from the 320 mg cohort of INTEGRIS-PSC, a multinational, randomized, double-blind, placebo-controlled Phase 2a clinical trial of bexotegrast in patients with primary sclerosing cholangitis (PSC) and suspected moderate to severe liver fibrosis. The 320 mg treatment group met its primary endpoint of safety, demonstrating that bexotegrast was well tolerated up to 40 weeks of treatment. Pruritus and cholangitis occurred in lower proportions on bexotegrast than on placebo, consistent with previous findings.
The trial’s exploratory efficacy endpoints assessed changes in liver stiffness as measured by transient elastography (TE) at 24 weeks, changes in the liver fibrosis markers including Enhanced Liver Fibrosis (ELF) score, as well as liver biochemistry and magnetic resonance imaging (MRI). Bexotegrast at 320 mg demonstrated improvement in liver stiffness compared to placebo at Week 24. A reduction in ELF score was observed at Week 24 in patients at higher risk of disease progression (baseline ELF > 9.8) compared to an increase in ELF on placebo. Stable ELF score was observed from Week 12 to Week 24 in the overall bexotegrast-treated population compared to placebo. Bexotegrast improved markers and symptoms of cholestasis including alkaline phosphatase (ALP), MRI, self-reported itch, and common adverse events associated with PSC. Bexotegrast-treated patients showed decreased ALP levels over 24 weeks, compared to increased ALP on placebo. MRI of the liver demonstrated evidence of further improvement of hepatocyte function and bile flow with bexotegrast at the 320 mg dose from Week 12 to 24.
INTEGRIS-PSC was a multinational, randomized, dose-ranging, double-blind, placebo-controlled Phase 2a trial designed to evaluate bexotegrast at once-daily oral doses of 40 mg, 80 mg, 160 mg or placebo up to 12 weeks and 320 mg or placebo for up to 48 weeks in 121 patients with PSC and suspected liver fibrosis. The 320 mg cohort enrolled 27 patients in the active arm and 9 patients in placebo arm.
“We could not ask for more from an exploratory Phase 2a study. These longer term INTEGRIS-PSC data continue to highlight bexotegrast’s favorable safety and tolerability profile, further validate its broad antifibrotic activity in diseases of unmet need and suggest the potential for disease stabilization,” said Éric Lefebvre, M.D., Chief Medical Officer of Pliant. “Furthermore, these positive data provide additional confidence in our IPF development program centered on BEACON-IPF, our ongoing global Phase 2b/3 trial.”
Bexotegrast 320 mg was Well Tolerated Up to Week 40 with No Drug-Related Severe or Serious Adverse Events
The primary endpoint of the INTEGRIS-PSC trial was the evaluation of the safety and tolerability of bexotegrast. Bexotegrast at 320 mg was well tolerated up to 40 weeks of treatment with no treatment-related severe or serious adverse events (SAE). Most treatment-emergent adverse events (TEAEs) were mild or moderate in severity and consistent with PSC disease symptoms.
Bexotegrast 320 mg Continued to Demonstrate Antifibrotic Activity in a PSC Population with Suspected Moderate to Severe Liver Fibrosis at Week 24
The exploratory endpoints of the INTEGRIS-PSC trial included changes in liver stiffness as measured by transient elastography (TE) at 24 weeks, changes in liver fibrosis markers including ELF, liver biochemistry and MRI imaging. These data suggest stabilization of liver fibrosis.
Bexotegrast at 320 mg demonstrated a numerical reduction in liver stiffness at Week 24 compared to an increase on placebo, as measured by TE. Liver stiffness is a marker of liver fibrosis that increases over time in patients with PSC. Measurement of liver stiffness by TE can be used to predict the severity and progression of liver fibrosis.1
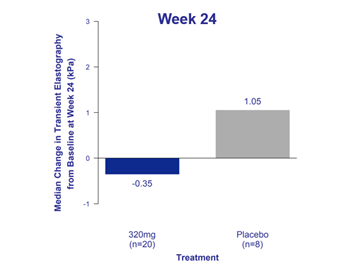
Figure 1. Liver Stiffness by Transient Elastography – Change from Baseline at Week 24
In patients at high risk for disease progression (baseline ELF ˃ 9.8), bexotegrast-treated patients showed a reduction in ELF score at Week 24 compared to an increase in placebo at Week 24. Across all bexotegrast-treated patients, ELF remained stable on treatment from Week 12 to 24 compared to placebo.
Bexotegrast Improved Markers and Symptoms of Cholestasis
Bexotegrast-treated patients demonstrated statistically significant reduction in ALP over 24 weeks compared to increased levels on placebo, with greater reductions observed in bexotegrast-treated patients with elevated baseline ALP values.
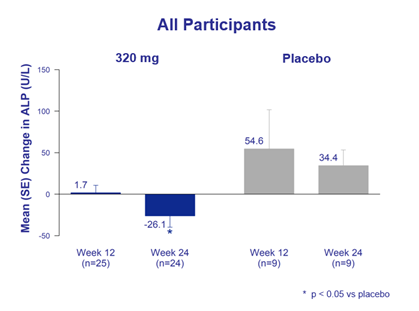
Figure 2. Alkaline Phosphatase – Change in Baseline Over 24 Weeks
At Week 12, in the MRI sub-study, bexotegrast-treated patients showed increased relative enhancement compared to decreased relative enhancement in the placebo group. At Week 24, bexotegrast-treated patients displayed a further increase in relative enhancement, suggesting continued improvement in hepatocyte function from Week 12 to 24. At Week 12, bexotegrast-treated patients showed decreased time to arrival to the common bile duct compared to placebo, suggesting improved bile flow.2 At Week 24, bexotegrast-treated patients showed faster time to arrival to the common bile duct, suggesting further improvement in bile flow from Week 12 to 24. Interpretation of placebo findings at Week 24 was limited due to the small number of placebo participants (n=˂2) enrolled in the MRI sub-study of the 320 mg cohort.
Pruritus and cholangitis are common symptoms of cholestasis in PSC patients.3 Adverse events of pruritus and cholangitis occurred in a lower proportion of bexotegrast-treated patients at 320 mg compared to placebo. Bexotegrast-treated patients demonstrated a stable score on the Itch Numerical Rating Scale relative to a numerical increase on placebo. These findings are consistent with previously reported data from Week 12 across all doses.
“I continue to be encouraged by the consistent safety and efficacy trends seen with these data that clearly align with the data previously presented,” said Gideon Hirschfield, FRCP, Ph.D., Lily and Terry Horner Chair in Autoimmune Liver Disease at the University of Toronto and a principal investigator in the INTEGRIS-PSC trial. “These data, alongside those from bexotegrast in IPF, suggest a strong clinical antifibrotic effect across multiple diseases. Today’s PSC data is further strengthened with the new transient elastography data in liver stiffness.”
Pliant would like to thank our INTEGRIS-PSC investigators and their study teams for their dedication in support of the successful execution of this trial. Special thanks to the INTEGRIS-PSC clinical trial participants, their families and support networks for helping us advance this promising program.
FDA Feedback Regarding Next Steps of the Development of Bexotegrast in PSC
Pliant recently conducted a meeting with U.S. Food and Drug Administration (FDA) to review the potential development path for bexotegrast in PSC. The FDA is supportive of a 52-week, dose-ranging Phase 2b trial employing non-invasive endpoints. Pliant will continue to evaluate the best path forward for this program.
Background on Primary Sclerosing Cholangitis
PSC is a rare, progressive liver disease of unknown origin, which frequently occurs in the setting of inflammatory bowel disease. PSC affects more than 30,000 patients in the United States and over 100,000 patients worldwide. The disease can occur in all ages, genders, and races. PSC is characterized by inflammation and fibrosis, with progressive liver and biliary damage leading to cirrhosis and liver failure. Currently there are no FDA or EMA-approved therapies for patients with PSC. Therefore, there is a high unmet need for new therapeutic options to address the symptoms and modify the disease progression of this grievous illness.
INTEGRIS-PSC Multinational Phase 2a Trial of Bexotegrast (NCT04480840)
INTEGRIS-PSC was a Phase 2a, multinational randomized, dose-ranging, double-blind, placebo-controlled trial evaluating the safety, tolerability, and pharmacokinetics of bexotegrast administered over 12 weeks in patients with PSC. Patients were enrolled in doses of 40 mg, 80 mg, 160 mg or 320 mg, with a 3:1 randomization ratio (active:placebo) and stratification based on use of ursodeoxycholic acid (UDCA). The primary endpoint was the evaluation of bexotegrast safety and tolerability, and the secondary endpoint is the assessment of pharmacokinetics across the range of doses. Exploratory endpoints measured changes in liver fibrosis markers, ELF and PRO-C3, liver biochemistry and liver imaging.
About Pliant Therapeutics, Inc.
Pliant Therapeutics is a late-stage biopharmaceutical company and leader in the discovery and development of novel therapeutics for the treatment of fibrotic diseases. Pliant's lead product candidate, bexotegrast (PLN-74809), is an oral, small molecule, dual selective inhibitor of αvß6 and αvß1 integrins that is in development in the lead indications for the treatment of idiopathic pulmonary fibrosis, or IPF, and primary sclerosing cholangitis, or PSC. Bexotegrast has received Fast Track Designation and Orphan Drug Designation from the U.S. Food and Drug Administration (FDA) in IPF and PSC and Orphan Drug Designation from the European Medicines Agency in IPF and PSC. Pliant has initiated BEACON-IPF, an adaptive Phase 2b/3 trial of bexotegrast in IPF. Pliant is conducting a Phase 1 study for its third clinical program, PLN-101095, a small molecule, dual-selective inhibitor of αvß8 and αvß1 integrins, that is being developed for the treatment of solid tumors. In addition, Pliant has received regulatory clearance for the conduct of a Phase 1 study of PLN-101325, a monoclonal antibody agonist of integrin α7β1 targeting muscular dystrophies.
For additional information, please visit: www.PliantRx.com. Follow us on social media X, LinkedIn, and Facebook.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as "may," "will," "expect," "anticipate," "estimate," "intend," and similar expressions (as well as other words or expressions referencing future events, conditions, or circumstances) are intended to identify forward-looking statements. These statements include those regarding the safety, tolerability, pharmacodynamics and therapeutic potential of bexotegrast; our plans for the future development of bexotegrast; bexotegrast’s potential to become a treatment for IPF or PSC; and discussions and interactions with regulatory authorities, including the FDA’s view of a 52-week, dose-ranging Phase 2b trial employing non-invasive endpoints. Because such statements deal with future events and are based on our current expectations, they are subject to various risks and uncertainties and actual results, performance or achievements of Pliant Therapeutics could differ materially from those described in or implied by the statements in this press release. These forward-looking statements are subject to risks and uncertainties, including those related to the development and commercialization of our product candidates, including any delays in our ongoing or planned preclinical or clinical trials, the impact of current macroeconomic and marketplace conditions, our reliance on third parties for critical aspects of our development operations, the risks inherent in the drug development process, the risks regarding the accuracy of our estimates of expenses and timing of development, our capital requirements and the need for additional financing, including the availability of additional term loans under our loan facility, and our ability to obtain and maintain intellectual property protection for our product candidates. These and additional risks are discussed in the sections titled "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" in our Quarterly Report on Form 10-Q for the period ended March 31, 2024, which is available on the SEC's website at www.sec.gov. Unless otherwise noted, Pliant is providing this information as of the date of this news release and does not undertake any obligation to update any forward-looking statements contained in this document as a result of new information, future events or otherwise.
Investor and Media Contact:
Christopher Keenan
Vice President, Investor Relations and Corporate Communications
Pliant Therapeutics, Inc.
IR@PliantRx.com
1 Corpechot C, et al. Gastroenterology. 2014 Apr;146(4):970-9.
2 Elkilany A, et al. Abdom Radiol (NY). 2021 Mar;46(3):979-991.
3 Karlsen TH, et al. J Hepatol. 2017 Dec;67(6):1298-1323.
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/a10eff0c-3a21-45a9-a983-45234737f34b
https://www.globenewswire.com/NewsRoom/AttachmentNg/dcac05b1-bda1-42d0-ac1f-fe52f0dc71f7






