- Reported 12-month overall survival (OS) in Phase 2a clinical trial nearly double the benchmark from pivotal study of standard of care gemcitabine/nab-paclitaxel (GnP) -
- Atebimetinib consistently demonstrates impressive overall survival advantage at all time periods reported; Median overall survival remains unreached as of data cutoff, suggesting encouraging durability of clinical benefit -
- Anticipate dosing first patient in pivotal Phase 3 clinical trial, MAPKeeper 301, in mid-2026 -
- Company to host conference call at 4:30 p.m. ET today -
NEW YORK, Jan. 07, 2026 (GLOBE NEWSWIRE) -- Immuneering Corporation (Nasdaq: IMRX), a late-stage clinical oncology company focused on keeping cancer patients alive and helping them thrive, today announced positive updated overall survival (OS) and safety data from its ongoing Phase 2a trial of atebimetinib (IMM-1-104) in combination with modified gemcitabine/nab-paclitaxel (mGnP) in first-line pancreatic cancer patients (N=34), with over 13 months median follow up time.
“We are thrilled to report 64% overall survival at 12 months in first-line pancreatic cancer patients treated with atebimetinib in combination with mGnP,” said Ben Zeskind, Ph.D., CEO of Immuneering. “The consistently strong separation observed between the overall survival in our clinical trial of atebimetinib in combination with mGnP in first-line pancreatic cancer patients and the benchmark for standard of care GnP from the MPACT study has now held at 6 months, 9 months, and 12 months. Having recently reached alignment on our planned pivotal Phase 3 clinical trial design with the FDA and EMA, with overall survival as our primary endpoint, we plan to dose the first patient in our MAPKeeper 301 pivotal trial in mid-2026, as we move to bring this new treatment option to patients as expeditiously as possible.”
Extraordinary Overall Survival (OS) Observed at 12 Months in First-Line Pancreatic Cancer

FOR ILLUSTRATIVE PURPOSES ONLY: No head-to-head clinical trial has been conducted evaluating atebimetinib and other candidates or products. Differences exist between trial designs, subject characteristics and other factors, and caution should be exercised when comparing data across studies. Reconstructed Kaplan-Meier (KM) Plot of Pivotal Ph3 Study MPACT 2013 NEJM (PMID: 24131140) per 2024 JAMA Nichetti, et al. 7(1):e2350756
- Consistently Strong Separation Observed in Overall Survival from Standard of Care. Atebimetinib (320mg dosed once-daily) + mGnP demonstrated remarkable OS at 12 months (median follow up of 13.4 months) in first-line pancreatic cancer patients (N=34), with the median OS not yet reached as of the data cutoff date of December 15, 2025. The MPACT pivotal trial for the standard of care benchmark, gemcitabine/nab-paclitaxel, reported significantly lower OS as noted below.
- 64% OS observed at 12 months; standard of care benchmark reported a 35% OS at 12 months.
- 83% OS observed at 9 months; standard of care benchmark reported a ~47% OS at 9 months.
- 94% OS observed at 6 months; standard of care benchmark reported a 67% OS at 6 months.
- Strong Separation Also Observed in Surrogate Endpoints from Standard of Care.
- Confirmed Overall Response Rate (ORR) of 39% at 12 months; standard of care benchmark reported an ORR of 23%.
- Disease Control Rate (DCR) of 81% at 12 months; standard of care benchmark reported a DCR of 48%.
- Median Progression Free Survival (mPFS) of 8.5 months; standard of care benchmark reported a mPFS of 5.5 months.
Unless otherwise specified, all data are reported using a data cutoff date of December 15, 2025, from the same patient cohort (N=34) as previously reported in September 2025. The estimates of (and other references to) standard of care set forth above with respect to the 6- and 12-month follow-up data were reported directly from the publicly available third-party MPACT pivotal trial data for gemcitabine/nab-paclitaxel. The estimates of (and other references to) standard of care with respect to the nine-month follow-up data were extrapolated and reconstructed by the Company based on the publicly available third-party MPACT pivotal trial data for gemcitabine/nab-paclitaxel. The Company’s Phase 1/2a clinical trial of atebimetinib does not include a head-to-head comparison against any other agents, and caution should be exercised when comparing data across trials.
The Company believes these compelling updated OS data reflect the potential for a durable, compounding benefit with atebimetinib + mGnP in first-line pancreatic cancer patients.
Continued Favorable Tolerability Profile Observed
As of the data cutoff date of December 15 2025, atebimetinib (320mg dosed once-daily) + mGnP continued to demonstrate a favorable tolerability profile in first-line pancreatic cancer patients (N=34), with only two categories of adverse events observed at the Grade 3 level in more than 10% of patients (neutropenia and anemia, both of which are categories commonly observed with standard of care chemotherapy and were previously noted in the September 2025 update). No new safety signals were identified.
Continued Favorable Tolerability Profile in First-Line Pancreatic Cancer
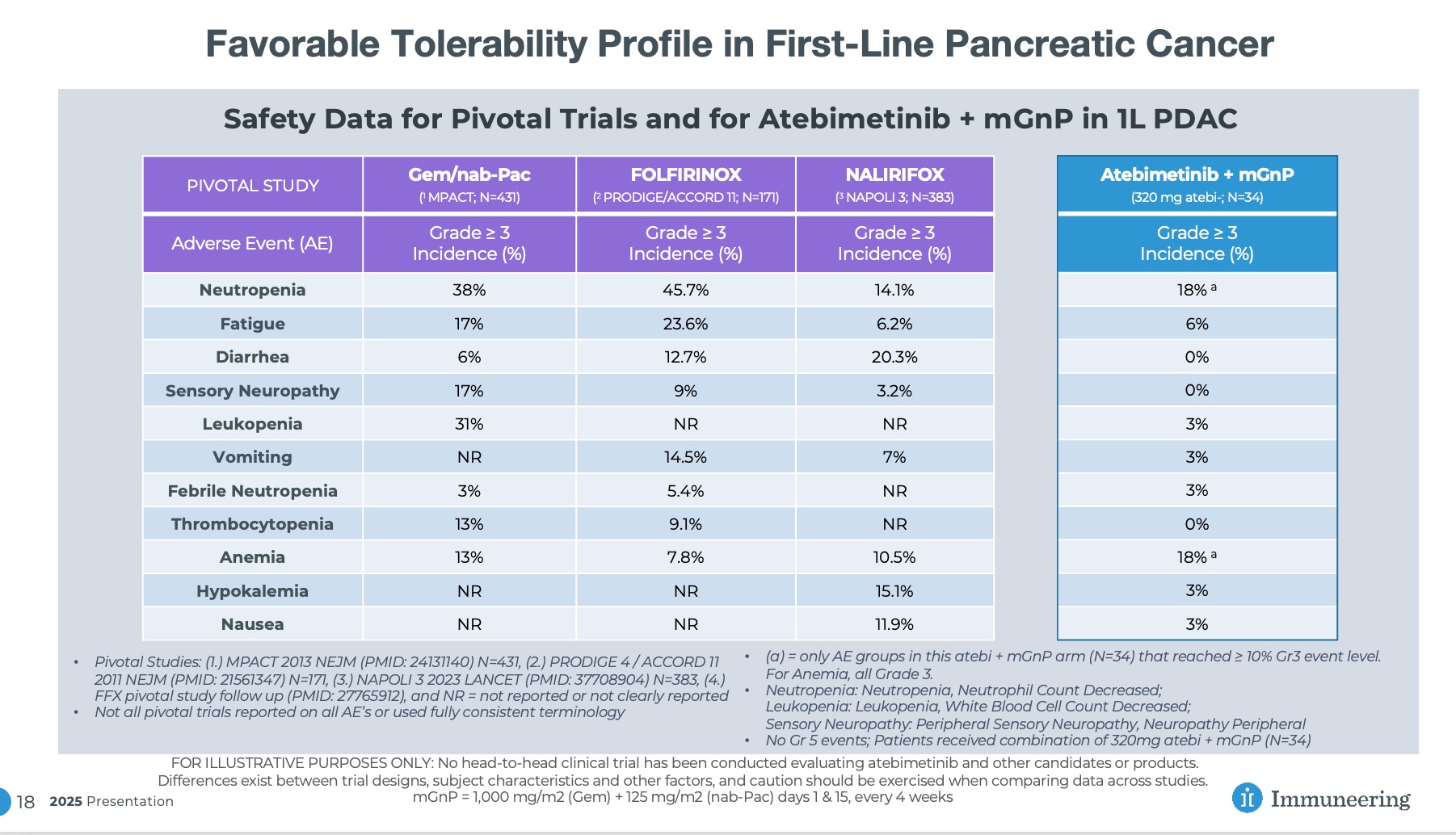
FOR ILLUSTRATIVE PURPOSES ONLY: No head-to-head clinical trial has been conducted evaluating atebimetinib and other candidates or products. Differences exist between trial designs, subject characteristics and other factors, and caution should be exercised when comparing data across studies. mGnP = 1,000 mg/m2 (Gem) + 125 mg/m2 (nab-Pac) days 1 & 15, every 4 weeks
“Based on the exceptional data from the ongoing Phase 2a clinical trial of atebimetinib in combination with mGnP in first-line pancreatic cancer, we believe atebimetinib has the potential to deliver extraordinary overall survival with both durability and tolerability, two patient-centered essentials that oncologists have long struggled to balance,” said Igor Matushansky, Chief Medical Officer at Immuneering. “Moving forward, in the first half of 2026 we plan to provide an update on an expanded cohort of over 50 first-line pancreatic cancer patients, which includes both the original 34 patients and additional patients we previously announced we planned to enroll, who are approaching sufficient median follow up time for presentation. We are excited to see that overall survival in the expanded cohort is trending consistently with what we have reported in the original 34 patients.”
“The overall survival data reported for atebimetinib demonstrate its potential to be a better treatment option for patients with pancreatic cancer, for whom there is a need for more durable and better-tolerated treatments,” said Dr. Meredith Pelster, Associate Director of Gastrointestinal Cancer Research for Sarah Cannon Research Institute, and investigator on the Phase 2a clinical trial. “Looking ahead, I share the enthusiasm of many in the field for the planned atebimetinib pivotal Phase 3 clinical trial and look forward to enrolling patients in the program."
Near-Term Milestone Expectations
Immuneering is planning for several near-term anticipated milestones related to atebimetinib, including:
- Q2 2026: Report updated circulating tumor DNA data on acquired alterations at a major scientific meeting.
- 1H 2026: Report updated survival data from over 50 first-line pancreatic cancer patients treated with atebimetinib + mGnP.
- Mid-2026: Dose first patient in pivotal Phase 3 clinical trial of atebimetinib in combination with mGnP in first-line pancreatic cancer.
- 2H 2026: Dose first patient in trial of atebimetinib in combination with Libtayo in non-small cell lung cancer.
Conference Call
Immuneering will host a conference call and live webcast at 4:30 p.m. ET / 1:30 p.m. PT on January 7, 2026, to discuss the data and provide a business update. Individuals interested in listening to the live conference call may do so by dialing (800) 715-9871 for U.S callers and (646) 307-1963 for other locations and reference conference ID 8438896, or from the webcast link in the “investors” section of the company's website at www.immuneering.com. A webcast replay will be available in the investor relations section on the company’s website for 90 days following the completion of the call.
About Atebimetinib
Atebimetinib is a Deep Cyclic Inhibitor (DCI), a new paradigm in targeted therapy. DCIs challenge the conventional model of sustained or continuous inhibition in oncology. Whereas most therapies are designed for sustained inhibition, driving cancer to adapt and develop resistance, so tumors shrink quickly but temporarily, DCIs are designed to pulse faster than tumors can adapt, so tumors shrink slowly but durably. Moreover, DCIs aim to restore full transient signaling to healthy cells, with the goal of leading to fewer adverse events. Atebimetinib targets MEK, a key control point in the MAPK pathway (RAS-RAF-MEK-ERK), which is pathologically activated in a majority of cancers, including approximately 97% of pancreatic cancers. Targeting MEK blocks a broader range of MAPK pathway alterations because it is further downstream, creating the potential for more durable benefit.
About Immuneering Corporation
Immuneering is a late-stage clinical oncology company focused on keeping cancer patients alive and helping them thrive. The Company is developing an entirely new category of cancer medicines, Deep Cyclic Inhibitors. Immuneering’s lead product candidate, atebimetinib, is an oral, once-daily Deep Cyclic Inhibitor of MEK, designed to improve durability and tolerability across many cancer indications, including MAPK pathway-driven tumors such as pancreatic cancer. Atebimetinib is currently planned to be evaluated in a Phase 3 trial in first-line pancreatic cancer, which is expected to begin dosing in mid-2026. The Company’s development pipeline also includes early-stage programs. For more information, please visit www.immuneering.com.
Forward-Looking Statements
This press release contains forward-looking statements, including within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including, without limitation, statements regarding: the treatment potential of atebimetinib, alone or in combination with other agents to treat cancer, including modified Gemcitabine/nab-paclitaxel (mGnP) in first-line pancreatic cancer, including its potential to deliver extraordinary overall survival with both durability and tolerability; the timing of commencing dosing in the Phase 3 trial; the ability of atebimetinib + mGnP to deliver a more durable and compounding benefit, including compared to standard of care; the timing, venue and content of future data releases and presentations and for the phase 2 results to continue to trend positively; and the timing for the initiation of additional atebimetinib clinical trial combination arms, including in non-small cell lung cancer.
These forward-looking statements are based on management’s current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the risks inherent in oncology drug research and development, including target discovery, target validation, lead compound identification, and lead compound optimization; we have incurred significant losses, are not currently profitable and may never become profitable; our projected cash runway; our need for additional funding; our unproven approach to therapeutic intervention; our ability to address regulatory questions and the uncertainties relating to regulatory filings, reviews and approvals; the lengthy, expensive, and uncertain process of clinical drug development, including potential delays in or failure to obtain regulatory approvals; our reliance on third parties and collaborators to conduct our clinical trials, manufacture our product candidates, and develop and commercialize our product candidates, if approved; failure to compete successfully against other drug companies; protection of our proprietary technology and the confidentiality of our trade secrets; potential lawsuits for, or claims of, infringement of third-party intellectual property or challenges to the ownership of our intellectual property; our patents being found invalid or unenforceable; costs and resources of operating as a public company; and unfavorable or no analyst research or reports.
These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q for the period ended September 30, 2025, and our other reports filed with the U.S. Securities and Exchange Commission, could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management's estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, except as required by law, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this press release.
Media Contact:
Carson Creehan
202-878-8330
Carson.creehan@padillaco.com
Investor Contact:
Courtney Dugan
917-971-3466
Cdugan@immuneering.com
Laurence Watts
619-916-7620
laurence@newstreetir.com
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/1168a985-0b95-416c-a580-d80bb6584779
https://www.globenewswire.com/NewsRoom/AttachmentNg/efce47fa-ef41-4495-a396-8e1e2bf09daf





