ABIOMED, Inc. - Common Stock (NQ:ABMD)
Press Releases about ABIOMED, Inc. - Common Stock
From Abiomed, Inc.
Via Business Wire







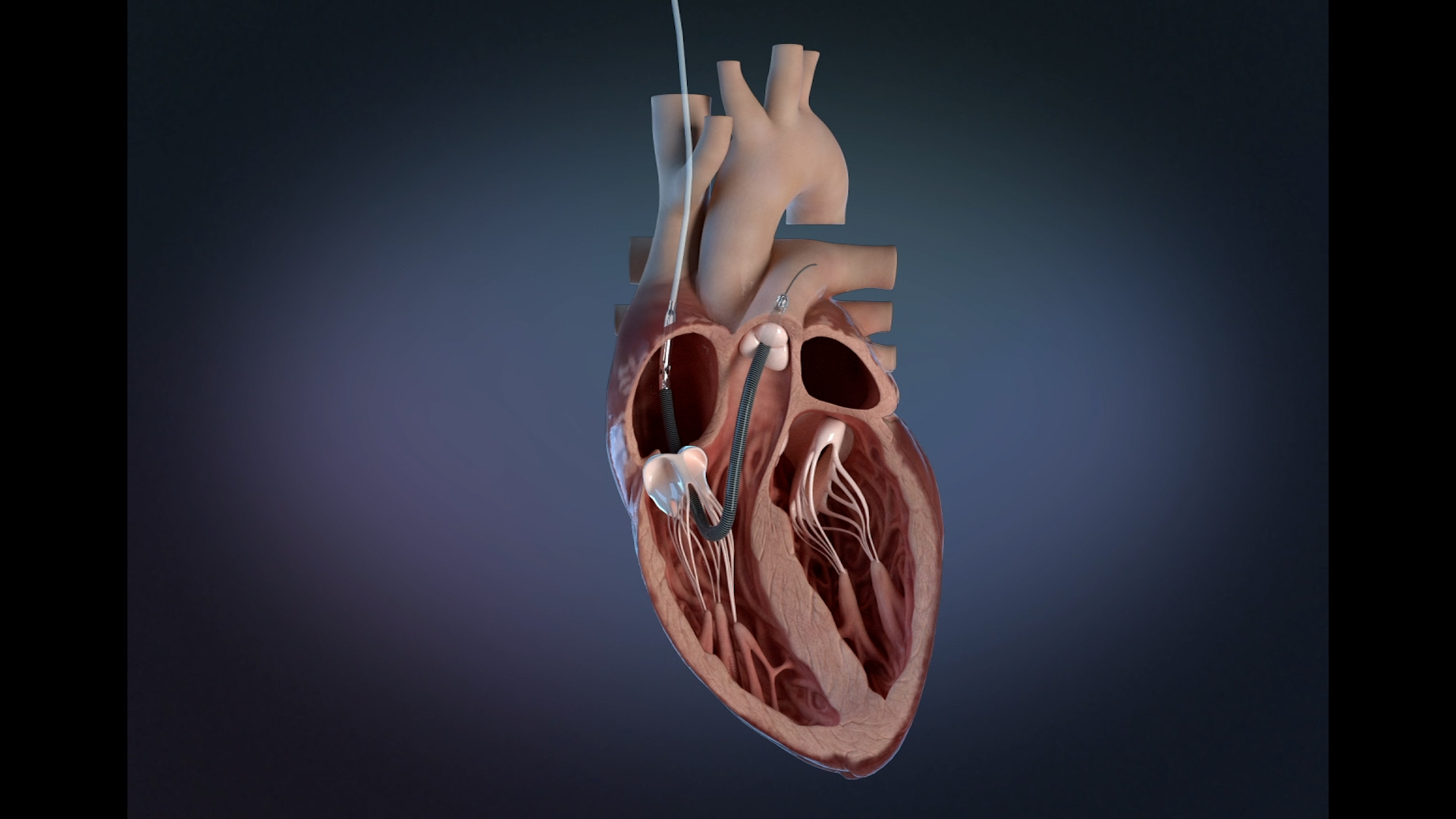
Impella RP Flex with SmartAssist Receives FDA Approval to Treat Right Heart Failure
October 31, 2022
From Abiomed, Inc.
Via Business Wire
From Abiomed, Inc.
Via Business Wire

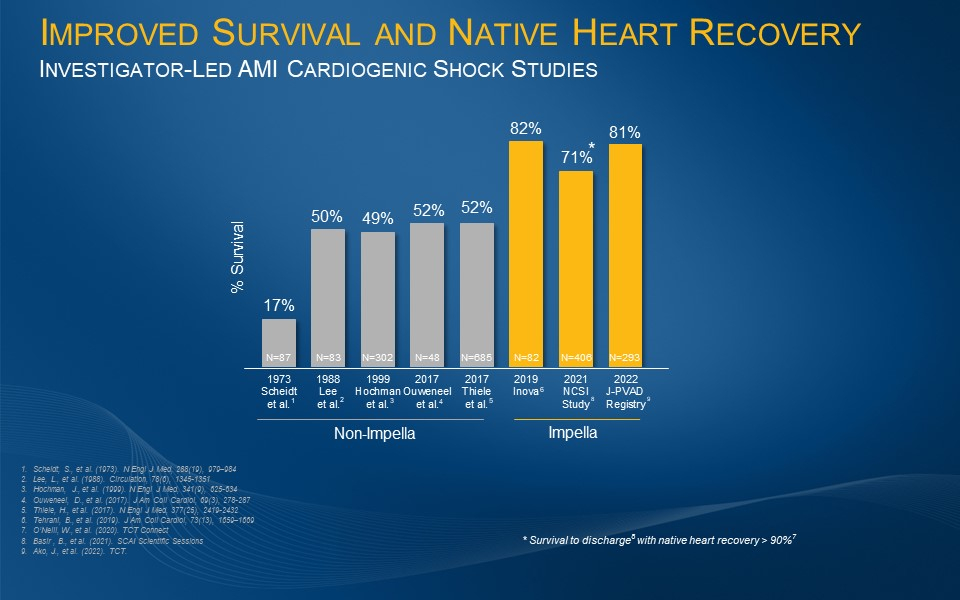
U.S. FDA Grants 510(k) Clearance for Impella Low Profile Sheath
October 17, 2022
From Abiomed, Inc.
Via Business Wire


From Abiomed, Inc.
Via Business Wire






Stock Quote API & Stock News API supplied by www.cloudquote.io
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.
© 2025 FinancialContent. All rights reserved.
