Arcturus Therapeutics Holdings Inc (NQ:ARCT)
Press Releases about Arcturus Therapeutics Holdings Inc









Arcturus Therapeutics to Attend Upcoming Investor Conferences
August 17, 2023
Via Business Wire

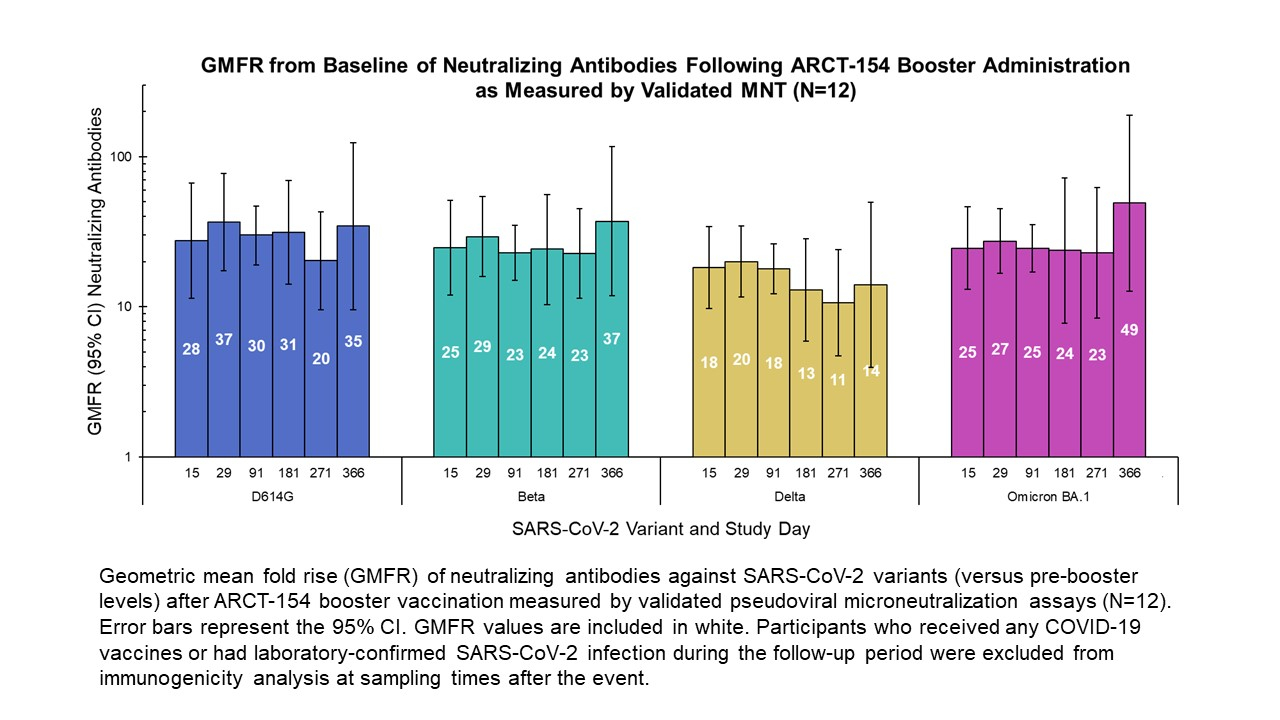







Arcturus Therapeutics to Attend Upcoming Investor Conferences
February 06, 2023
Via Business Wire



Arcturus Appoints John Markels, Ph.D. to its Board of Directors
December 13, 2022
Via Business Wire

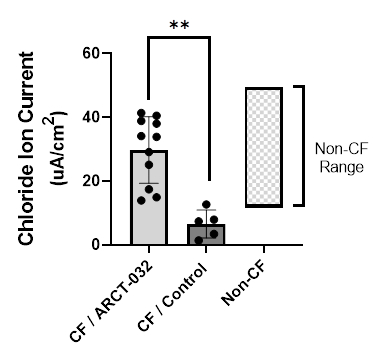




Arcturus Therapeutics to Attend the Following Investor Conferences
September 27, 2022
Via Business Wire

Arcturus Therapeutics to Attend the Following Investor Conferences
September 01, 2022
Via Business Wire
Stock Quote API & Stock News API supplied by www.cloudquote.io
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.
© 2025 FinancialContent. All rights reserved.
