Articles published by Arcturus Therapeutics Holdings Inc.



Via Business Wire

Via Business Wire
Tickers
ARCT

Arcturus Therapeutics Announces Second Quarter 2024 Financial Update and Pipeline Progress
August 05, 2024
Via Business Wire
Tickers
ARCT

Via Business Wire
Tickers
ARCT



Via Business Wire
Tickers
ARCT


Via Business Wire
Tickers
ARCT

Via Business Wire
Tickers
ARCT




Arcturus Therapeutics to Present at the Barclays 26th Annual Global Healthcare Conference
February 26, 2024
Via Business Wire
Tickers
ARCT



Arcturus Therapeutics to Present at the 42nd Annual J.P. Morgan Healthcare Conference
December 04, 2023
Via Business Wire
Tickers
ARCT



Arcturus Therapeutics Announces Third Quarter 2023 Financial Update and Pipeline Progress
November 14, 2023
Via Business Wire
Tickers
ARCT


Via Business Wire
Tickers
ARCT



Study Shows Novel sa-mRNA Vaccines Offer Robust, Broad, Enduring Protection Against COVID-19 Variants
September 19, 2023
Via Business Wire
Tickers
ARCT

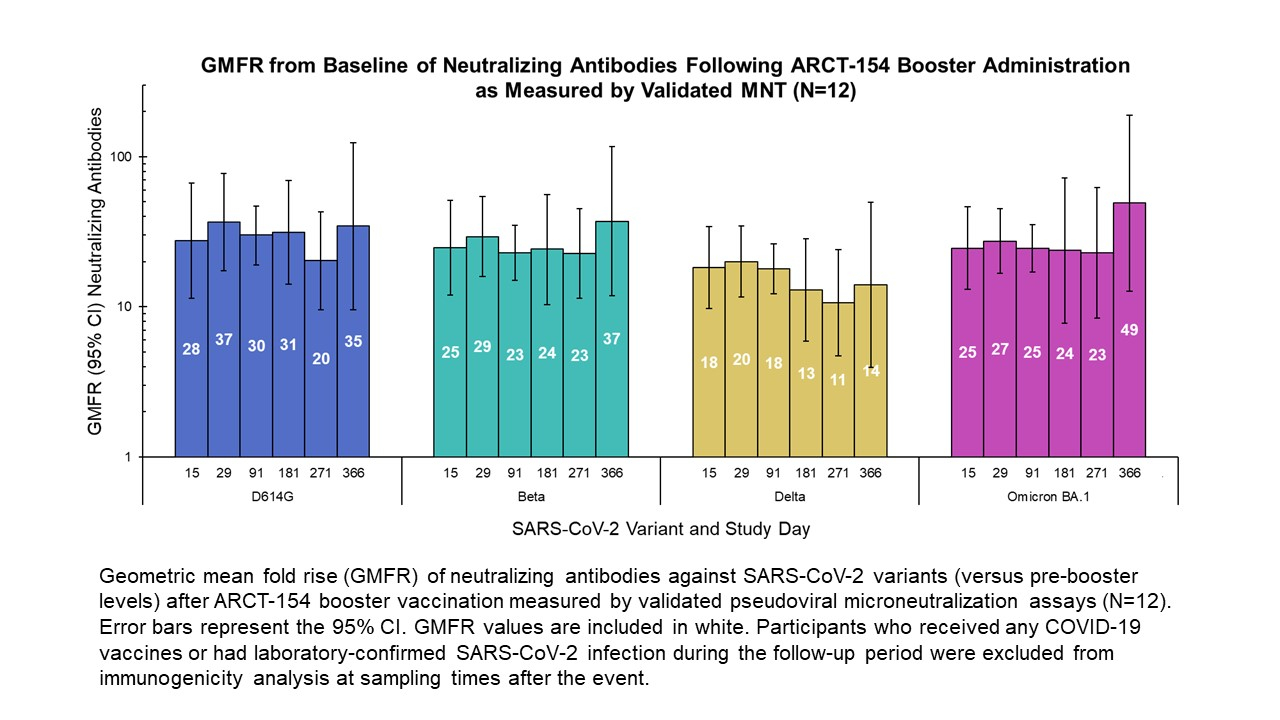
Arcturus Therapeutics Announces Second Quarter 2023 Financial Update and Pipeline Progress
August 07, 2023
Via Business Wire
Tickers
ARCT
Stock Quote API & Stock News API supplied by www.cloudquote.io
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.
© 2025 FinancialContent. All rights reserved.
