Creative Diagnostics, a reagent supplier and developer focused on biologics quality control, is excited to announce the launch of its Mammalian DNA Residue Assay Kits (qPCR) to the research community for precise and reliable detection and quantification of residual mammalian host cell DNA in biopharmaceutical products, ensuring stringent quality control and regulatory compliance.
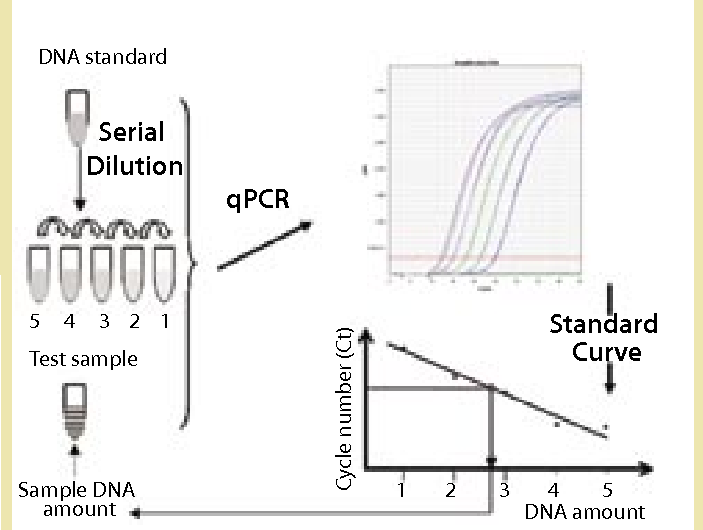
Detection of residual DNA in host cells throughout the biopharmaceutical manufacturing process is an important step in ensuring product safety and batch validation. Among the methods for detecting residual DNA, qPCR is considered the most practical method due to its sensitivity, accuracy, precision and time-saving. Creative Diagnostics now offers a comprehensive range of Mammalian DNA Residue Assay Kits (qPCR) to accurately detect and quantify residual mammalian host cell DNA throughout the biopharmaceutical manufacturing process, including the Vero DNA Residue Assay Kit, MDCK DNA Residue Assay Kit, CV-1 DNA Residue Assay Kit, CHO DNA Fragment Residue Assay Kit, and BHK DNA Residue Assay Kit.
For example, the MDCK DNA Residue Assay Kit is based on the principle of the PCR fluorescent probe method for the quantification of MDCK residual DNA in samples, which is fast, specific and reliable with a minimal limit of detection for fg levels. The kit is supplied with a reference standard for the quantification of MDCK DNA and can be used in conjunction with the Creative Diagnostics Host Cell DNA Pretreatment Kit to accurately determine the amount of residual MDCK DNA in biological samples.
Another example is the HEK293 DNA Fragment Residue Assay Kit. Using the PCR fluorescent probe method, four different amplification fragments (75 bp, 122 bp, 244 bp, 562 bp) have been designed to quantitatively analyze the size distribution of residual HEK293 DNA fragments in samples. The kit is supplied with a reference standard for HEK293 DNA quantification. It can be used in combination with the Creative Diagnostics Host Cell DNA Pretreatment Kit to accurately determine the amount of residual HEK293 DNA in biological samples.
By leveraging these advanced assay kits, biopharmaceutical manufacturers can significantly improve their operations by reducing product safety risks associated with residual host cell DNA, improving product quality and consistency, ensuring compliance with stringent regulatory guidelines, and optimizing downstream purification processes. These kits represent a significant advancement in biopharmaceutical quality control, enabling professionals to produce safe and effective products that meet the highest regulatory standards.
Creative Diagnostics is dedicated to supporting the biopharmaceutical industry with comprehensive quality control solutions. The company offers a full range of products and services, including assay kits, reagents and custom services, to meet the diverse needs of researchers and manufacturers. For more information about Mammalian DNA Residue Assay Kits and other product offerings, please visit https://qbd.creative-diagnostics.com/products/mammalian-dna-residue-assay-kits-qpcr-2747.html.
About Creative Diagnostics
Creative Diagnostics is a global leader in the development and manufacturing of innovative tools and reagents for bioprocess impurity analysis. The company offers a comprehensive portfolio of solutions to support researchers in the quality control of biologics and provides biopharmaceutical quality, purity and safety assays, analytical methods and applications for the biotechnology and biopharmaceutical industries.
Media Contact
Company Name: Creative Diagnostics
Contact Person: Thomas Schmitt
Email: Send Email
State: New York
Country: United States
Website: https://qbd.creative-diagnostics.com/