The TRUFORMA Platform now has the widest dynamic range (without manual dilution) available either at the Point of Care or from a Reference Lab
ANN ARBOR, MI / ACCESS Newswire / May 21, 2025 / Zomedica Corp. (OTCQB:ZOMDF) ("Zomedica" or the "Company"), a veterinary health company offering point-of-care diagnostics and therapeutic products for equine and companion animals, today announced an update to one of their fastest growing assays, insulin for equine plasma. This update increases the test's already industry-leading point of care dynamic range and adds a new function, automatic sample dilution, that allows a veterinarian to measure insulin at the highest levels with no additional steps or increase in time-to-result on the TRUFORMA In-Clinic Biosensor Testing Platform.
"When we introduced our equine insulin assay in October of 2024, one of the most frequent requests we received was for a dilution protocol to measure insulin level in horses suspected to be greater than 250 µU/mL," said Ian Harmon, Senior Director, R&D. Ian continues, "In response, rather than validating an external dilution protocol that would involve additional steps for the veterinarian and staff, our engineers designed a way to have the TRUFORMA device automatically dilute the sample for them and run the test with no additional time or steps. The user simply selects the ‘Auto Dilute' option from the test menu screen, and the TRUFORMA device takes it from there. The uniqueness of our single-use test cartridges allows us this flexibility of design and is something that no other platform can do."
By having the TRUFORMA device perform the dilution automatically on the existing test cartridge, consistency from test-to-test is assured and the staff is freed from having to perform a tedious additional task.
The Zomedica R&D team was also able to increase the already industry-leading dynamic range of the standard (or default, undiluted) in-clinic insulin test by 60%! The chart below illustrates the new dynamic ranges for both the default and auto dilute protocols, making the TRUFORMA insulin test for equine plasma the most dynamic test of its kind in the world.
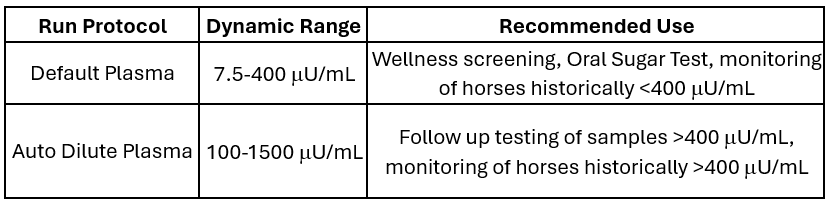
T.J. Barclay, DVM, Senior Professional Services Veterinarian for Zomedica, commented, "In recent years, insulin dysregulation in horses and ponies has been increasingly recognized as the most significant risk factor for developing laminitis. In the more severe cases, insulin levels may be above a test's upper limit of detection, rendering us unable to determine by serial monitoring if our treatment is effective. In those cases, quantifying extremely high insulin levels has required sending samples to a reference lab where they can perform sample dilutions. Having a device that gives us results in minutes at the point of care that has a both a high dynamic range by default and a process that dilutes the sample on demand covers all patient types we may encounter, helping us make quicker decisions during the treatment and monitoring process."
With the increased dynamic range and new automatic dilution function, along with the consistency, accuracy, and convenience of the original assay, Zomedica has set a new standard for point of care equine insulin testing.
The TRUFORMA Insulin assay for equine plasma with automatic sample dilution may be ordered now from Zomedica. For more information, visit www.zomedica.com.
About Zomedica
Zomedica is a leading equine and companion animal healthcare company dedicated to improving animal health by providing veterinarians with innovative therapeutic and diagnostic solutions. Our gold standard PulseVet® shock wave system, which accelerates healing in musculoskeletal conditions, has transformed veterinary therapeutics. Our suite of products also includes the Assisi® Loop line of therapeutic devices and the TRUFORMA® diagnostic platform, the TRUVIEW® digital cytology system, and the VetGuardian® no-touch monitoring system, all designed to empower veterinarians to provide top-tier care. In the aggregate, their total addressable market in the U.S. exceeds $2 billion. Headquartered in Michigan, Zomedica employs approximately 150 people and manufactures and distributes its products from its world-class facilities in Georgia and Minnesota. Zomedica grew revenue 8% in 2024 to $27 million and maintains a strong balance sheet with approximately $65 million in liquidity as of March 31, 2025. Zomedica is advancing its product offerings, leveraging strategic acquisitions, and expanding internationally as we work to enhance the quality of care for pets, increase pet parent satisfaction, and improve the workflow, cash flow and profitability of veterinary practices. For more information visit www.zomedica.com.
Follow Zomedica
Email Alerts: http://investors.zomedica.com
Facebook: https://m.facebook.com/zomedica
X (formerly Twitter): https://twitter.com/zomedica
Instagram: https://www.instagram.com/zomedica_inc
Cautionary Note Regarding Forward-Looking Statements
Except for statements of historical fact, this news release contains certain "forward-looking information" or "forward-looking statements" (collectively, "forward-looking information") within the meaning of applicable securities law. Forward-looking information is frequently characterized by words such as "plan", "expect", "project", "intend", "believe", "anticipate", "estimate" and other similar words, or statements that certain events or conditions "may" or "will" occur and include statements relating to our expectations regarding future results. Although we believe that the expectations reflected in the forward-looking information are reasonable, there can be no assurance that such expectations will prove to be correct. We cannot guarantee future results, performance, or achievements. Consequently, there is no representation that the actual results achieved will be the same, in whole or in part, as those set out in the forward-looking information.
Forward-looking information is based on the opinions and estimates of management at the date the statements are made, including assumptions with respect to economic growth, demand for the Company's products, the Company's ability to produce and sell its products, sufficiency of our budgeted capital and operating expenditures, the satisfaction by our strategic partners of their obligations under our commercial agreements and our ability to realize upon our business plans and cost control efforts.
Our forward-looking information is subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those anticipated in the forward-looking information. Some of the risks and other factors that could cause the results to differ materially from those expressed in the forward-looking information include, but are not limited to: the outcome of clinical studies, the application of generally accepted accounting principles, which are highly complex and involve many subjective assumptions, estimates, and judgments, uncertainty as to whether our strategies and business plans will yield the expected benefits; uncertainty as to the timing and results of development work and verification and validation studies; uncertainty as to the timing and results of commercialization efforts, including international efforts, as well as the cost of commercialization efforts, including the cost to develop an internal sales force and manage our growth; uncertainty as to our ability to realize the anticipated growth opportunities from our acquisitions; uncertainty as to our ability to supply products in response to customer demand; supply chain risks associated with tariff changes;; uncertainty as to the likelihood and timing of any required regulatory approvals, and the availability and cost of capital; the ability to identify and develop and achieve commercial success for new products and technologies; veterinary acceptance of our products and purchase of consumables following adoption of our capital equipment; competition from related products; the level of expenditures necessary to maintain and improve the quality of products and services; changes in technology and changes in laws and regulations; our ability to secure and maintain strategic relationships; performance by our strategic partners of their obligations under our commercial agreements, including product manufacturing obligations; risks pertaining to permits and licensing, intellectual property infringement risks, risks relating to any required clinical trials and regulatory approvals, risks relating to the safety and efficacy of our products, the use of our products, intellectual property protection, and the other risk factors disclosed in our filings with the SEC and under our profile on SEDAR+ at www.sedarplus.com. Readers are cautioned that this list of risk factors should not be construed as exhaustive.
The forward-looking information contained in this news release is expressly qualified by this cautionary statement. We undertake no duty to update any of the forward-looking information to conform such information to actual results or to changes in our expectations except as otherwise required by applicable securities legislation. Readers are cautioned not to place undue reliance on forward-looking information.
Investor Relations Contact:
Zomedica Investor Relations
investors@zomedica.com
1-734-369-2555
SOURCE: Zomedica Corp.
View the original press release on ACCESS Newswire




