Opioid Halo Is Indicated for the Detection of Opioid-Induced Respiratory Depression, with Escalating Alarms to Alert Loved Ones That Help Is Needed
De Novo Authorizes Both Over-the-Counter (OTC) Use, on Adults and Children Age 15 and Up, and Prescription Use
Masimo (NASDAQ: MASI) today announced that Masimo Opioid Halo™, an opioid overdose prevention and alert system, has been granted a De Novo by the FDA – making it the first and only FDA-cleared monitoring solution for detecting opioid-induced respiratory depression, the leading cause of death from opioid overdose. With the De Novo, Masimo also becomes the first winner of an FDA Opioid Innovation Challenge to have an authorized solution designed to help solve the U.S. opioid crisis. The De Novo authorizes Opioid Halo to be made available over the counter (OTC) without a prescription, for use on adults and children age 15 and up, and an Rx version for use by prescription from a healthcare provider. Opioid Halo advances the forefront of continuous monitoring through its unique Opioid Halo engine, an advanced pattern recognition algorithm which helps detect and quantify the risk of severe opioid-induced respiratory depression. Combined with its innovative distributed architecture, Opioid Halo helps to manage and send escalating alarms to family members, friends, and caregivers, notifying them that help may be needed due to an opioid overdose – including triggering an automatic wellness call, which may lead to EMS being dispatched.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230403005131/en/
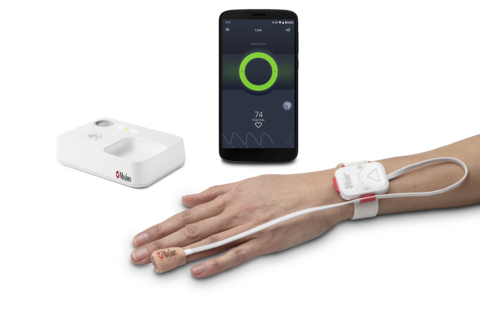
Masimo Opioid Halo™ (Photo: Business Wire)
Joe Kiani, Founder and CEO of Masimo, said, “We are very excited to be able to offer this solution to our fellow Americans and the community heroes who are helping to battle the opioid crisis – a crisis so devastating in its impact on the young that it has lowered overall life expectancy in the U.S. Now, with Opioid Halo, we hope to help make a big difference by providing a much needed tool that can help millions, whether they are taking prescribed opioids or struggling with illicit opioid use. In 2018, we were honored to be chosen by the FDA, based on our expertise in patient monitoring technologies, to develop a device that could help prevent opioid overdose, as part of their Innovation Challenge addressing the opioid epidemic. And today, we are delighted to have received the first De Novo for our response to that Innovation Challenge, Masimo Opioid Halo. Between then and now, the COVID-19 pandemic accelerated our development and refinement of the Masimo SafetyNet® family of remote patient management and telehealth solutions, helping save many COVID patients’ lives. We thank the FDA for taking on the opioid epidemic and granting this De Novo – a clearance that is a huge step forward in preventing overdose deaths and helping to end the opioid crisis.”
Opioid overdose is the leading cause of accidental death in the US, responsible for more than 80,000 of the approximately 100,000 drug-related deaths in 2021, from both illicit opioids, such as fentanyl and heroin, and prescription opioids. Hundreds of thousands more suffer non-fatal overdose events, or the loss of a family member or friend to opioids.1 Opioids are readily prescribed in the US, with more than 143 million opioid prescriptions written in 2020,2 because they can be an effective way to help people manage pain, including after surgery or for chronic conditions. However, opioids also carry serious side effects, especially opioid-induced respiratory depression – slowed or stopped breathing –which is the leading cause of death from opioid overdoses.3 Anyone taking opioids, prescription or illicit, is at risk of experiencing an accidental overdose, the signs of which are unpredictable and can be difficult to detect.
Masimo Opioid Halo is designed to help family and friends identify the symptoms of an opioid overdose by detecting physiological markers present during opioid-induced respiratory depression and ideally, helping them know when it’s time to intervene – for example, by administering a potentially life-saving dose of naloxone. Opioid Halo can be used at home or in the hospital or another care setting, by patients prescribed opioids after surgery or managing a chronic or prolonged condition, as well as people suffering from opioid use disorder.
The Opioid Halo system consists of four components: 1) a tetherless, adhesive fingertip sensor; 2) a reusable Masimo SET® pulse oximeter and Bluetooth® chip; 3) a Bluetooth-to-Wi-Fi Masimo Home Medical Hub; and 4) a smartphone app. The fingertip sensor provides real-time monitoring for opioid-induced respiratory depression, enabled by the Opioid Halo pattern recognition algorithm and Masimo SET®, Signal Extraction Technology® – even during movement, when hands are cold, and on all skin pigmentations. Data from the sensor and chip are wirelessly relayed to the Masimo Home Medical Hub and the smartphone app, which continuously analyzes the user’s physiological data for trends and patterns associated with the physiology of an opioid-induced respiratory depression event to quantify the risk of an opioid overdose. As the level of risk rises, the app and hub provide alerts. Upon early onset, an audible and visual alarm, designed to trigger early intervention opportunities for the user to self-recover or get help, is provided. If the Opioid Halo risk score continues to worsen, in addition to the repeated alarms, automatic texts are sent to designated friends and family members, letting them know it may be time to intervene, for example by administering naloxone or taking other action. Finally, if the severity of the risk level progresses even further, there is an optional setting that can be activated during setup that enables a service center to place an automatic wellness call to the user, the outcome of which may lead to EMS being dispatched.
Kim Bennion, MsHS, RRT, CHC, FAARC, System Director of Research for Respiratory Care Clinical Services at Intermountain Health, a 33-hospital system based in Salt Lake City, Utah, said, “Painkillers often called ‘opioids’ can have negative side effects, including opioid-induced respiratory depression. Intermountain Health has demonstrated that home monitoring of post-surgical patients receiving opioids for pain can help identify issues earlier and avoid adverse outcomes. As a registered respiratory therapist and leader in the field of opioid-induced respiratory depression in the post-surgical setting, we are excited that Masimo’s breakthrough device will be available for our patients.”
Ryan Hampton, a noted advocate, author, and person in recovery, said, “Joe Kiani and his team at Masimo are strong advocates for solutions to help end the overdose crisis. Opioid Halo is another breakthrough innovation that can help save lives by adding to the critical support tools for people who use drugs.”
“My son Parker lost his life after taking prescription opioids to manage his pain after a tonsillectomy,” said Yvonne Gardner, now a patient safety advocate. “No one warned us that this could happen. This technology can help save other parents from losing a child.”
Masimo CEO Kiani added, “For more than 30 years, Masimo has been empowering clinicians with life-saving technologies that improve outcomes for countless patients around the world. Opioid Halo furthers our mission to expand beyond the hospital and into the home, empowering everyday people with a solution that we believe has the power to improve and save many more lives.”
For more information about Masimo Opioid Halo, and to pre-order it today for a loved one, a community hero, or an organization, visit opioidhalo.com.
@Masimo | #Masimo
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.4 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,5 improve CCHD screening in newborns6 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.7-10 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,11 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.12 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius Tº® and the Masimo W1™ watch. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi, RPVi, and Radius VSM have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm
- https://www.cdc.gov/drugoverdose/rxrate-maps/index.html
- Schiller EY, Goyal A, Mechanic OJ. Opioid Overdose. [Updated 2022 Sep 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470415/
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Opioid Halo™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Opioid Halo, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230403005131/en/
Contacts
Masimo
Evan Lamb
949-396-3376
elamb@masimo.com



