VANCOUVER, British Columbia, May 28, 2025 (GLOBE NEWSWIRE) -- BetterLife Pharma Inc. (“BetterLife” or the “Company”) (CSE: BETR / OTCQB : BETRF / FRA: NPAU), an emerging biotech company focused on the development of BETR-001, a non-hallucinogenic neuroplastogen in the treatment of psychiatric and neurological disorders, recently presented a scientific update at Bloom Burton Conference on May 5, 2025 in Toronto, Canada.
The emerging new paradigm in treating psychiatric and neurological disorders is the ability of a drug to enable the brain to rewire by growing new dendrites and spines, which enables new connections between neurons. This phenomenon is called neuroplasticity and the drug that is able to induce such an effect is called a neuroplastogen. BetterLife reported data at the Bloom Burton Conference showing that BETR-001 is a potent neuroplastogen. The figure below shows images of rat brain cortical neurons in vitro treated with either BETR-001, or ketamine (as a comparator), or no drug. Only BETR-001 treatment was able to significantly induce formation of new spines (green stain) in cultured neurons.
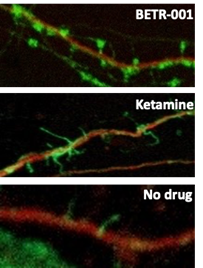
FIGURE: Growth of new neuronal spines in rat brain cortical neuron cultures treated with either BETR-001, ketamine or no drug.
In additional experiments, BETR-001 was also shown to significantly increase both the number and length of the neuronal dendrites, as exemplified in the data shown in the figure below.
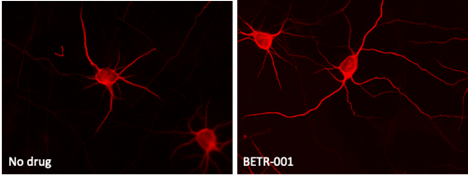
FIGURE: Growth of new neuronal dendrites in rat brain cortical neuron cultures treated with either no drug or BETR-001.
More detailed data and background on the neuroplastogenic activity of BETR-001 can be found in the peer-reviewed publication Lewis et al., Cell Reports, 2023.
Based on its excellent neuroplasticity data, its neuroreceptor activation profile and preliminary data obtained in animal models, BETR-001 is projected to be effective in treating various psychiatric and neurological disorders including depression, anxiety, PTSD, traumatic brain injury, migraines and neuropathic pain.
BETR-001 is non-hallucinogenic and not a controlled substance. BetterLife has been granted a composition of matter patent on BETR-001 by the USPTO (BetterLife press release January 14, 2025). BetterLife has had its BETR-001 pre-IND meeting with the FDA and has completed most of the required IND-enabling studies. The BETR-001 IND filing and start of human trials are projected for H1 2026.
About BetterLife Pharma
BetterLife Pharma Inc. is an emerging biotechnology company primarily focused on developing and commercializing two compounds, BETR-001 and BETR-002, to treat neuro-psychiatric and neurological disorders.
BETR-001, which is in preclinical and IND-enabling studies, is a non-hallucinogenic neuroplastogen. BETR-001 is a non-controlled substance. BETR-001 will be developed for the treatment of various psychiatric and neurological disorders. BETR-001 pending patent, for composition and method of use, covers treatment of major depressive disorder, anxiety disorder and neuropathic pain and other neuro-psychiatric and neurological disorders.
BETR-002, which is in preclinical and IND-enabling studies, is based on honokiol, the active anxiolytic ingredient of magnolia bark. BetterLife’s pending method of use and formulations patent covers treatment of anxiety-related disorders including benzodiazepine dependency.
BetterLife also owns a drug candidate for the treatment of viral infections and is in the process of seeking strategic alternatives for further development.
For further information, please visit BetterLife Pharma.
Contact
David Melles, Investor Relations Manager
Email: David.Melles@blifepharma.com
Phone: 1-778-887-1928
Cautionary Note Regarding Forward-Looking Statements
No securities exchange has reviewed nor accepts responsibility for the adequacy or accuracy of the content of this news release. This news release contains forward-looking statements relating to product development, licensing, commercialization and regulatory compliance issues and other statements that are not historical facts. Forward-looking statements are often identified by terms such as “will”, “may”, “should”, “anticipate”, “expects” and similar expressions. All statements other than statements of historical fact, included in this release are forward-looking statements that involve risks and uncertainties. There can be no assurance that such statements will prove to be accurate and actual results and future events could differ materially from those anticipated in such statements. Important factors that could cause actual results to differ materially from the Company’s expectations include the failure to satisfy the conditions of the relevant securities exchange(s) and other risks detailed from time to time in the filings made by the Company with securities regulations. The reader is cautioned that assumptions used in the preparation of any forward-looking information may prove to be incorrect. Events or circumstances may cause actual results to differ materially from those predicted, as a result of numerous known and unknown risks, uncertainties, and other factors, many of which are beyond the control of the Company. The reader is cautioned not to place undue reliance on any forward-looking information. Such information, although considered reasonable by management at the time of preparation, may prove to be incorrect and actual results may differ materially from those anticipated. Forward-looking statements contained in this news release are expressly qualified by this cautionary statement. The forward-looking statements contained in this news release are made as of the date of this news release and the Company will update or revise publicly any of the included forward-looking statements as expressly required by applicable law.
Figures accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/c7ea51b6-3470-4e08-aba9-e346d59414d8
https://www.globenewswire.com/NewsRoom/AttachmentNg/be2b1d49-f104-4211-8c6e-b1f005439b22





